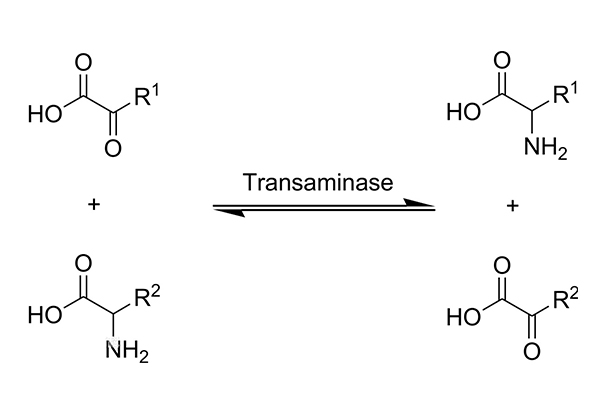
Amino acids contain amine (NH2) groups. Keto acids contain a ketone (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on another molecule. Amino acids become keto acids, and keto acids become amino acids.
Most transaminases are protein enzymes. However, some transamination activities of the ribosome have been found to be catalyzed by ribozymes (RNases). Examples are hammerhead ribozymes, VS ribozymes and hairpin ribozymes.
Transaminases require the coenzyme pyridoxal-phosphate, which is converted to pyridoxamine in the first stage of the reaction when amino acids are converted to ketoacids. The enzyme-bound pyridoxamine in turn reacts with pyruvate, oxaloacetate, or alpha-ketoglutarate to yield alanine, aspartate, or glutamate, respectively. Many transamination reactions take place in tissues and are catalyzed by transaminases specific for specific amino/keto acid pairs. The reaction is easily reversed, and the direction is determined by which reactant is in excess. For example, a specific enzyme comes from one of the reactant pairs; the reaction between glutamate and pyruvate, which makes alpha-ketoglutarate and alanine known as glutamate-pyruvate transaminase or GPT for short.
Tissue aminotransferase activity can be studied by incubating homogenates with various amino/keto acid pairs. Transamination is demonstrated if the formation of the corresponding new amino acids and ketoacids is shown by paper chromatography. Reversibility was demonstrated by using complementary ketone/amino acid pairs as starting reactants. After removing the chromatogram from the solvent, the chromatogram was treated with ninhydrin to locate the spots.
 Contact us
Contact us